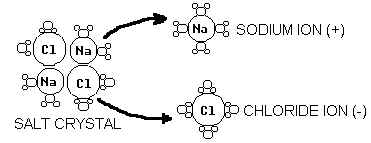
1. Draw a diagram that shows how water dissolves table salt (NaCl).
2. particulates; sugars, starches, proteins and other organic molecules; dissolved gases
3. 3.5% or 35%o or 35 parts per thousand (ppt)
4. Salinity increases when fresh water is removed from the ocean by evaporation or freezing. Salinity decreases when fresh water is added to the ocean by runoff, precipitation and ice melting. All of these processes happen at the surface of the ocean.
5. Weathering leaches salts from rocks on land which are carried into the ocean by streams. Volcanic activity releases volatiles like Cl2, Br2 and I2 into the atmosphere, which enter the ocean dissolved in rain. Sea floor hot springs also contribute ions.
6. The most abundant ions in the ocean are not the most abundant ions in river water. Complex reactions in the ocean remove some of the river water ions, while others are retained. Excess volatiles like chlorine are added directly via volcanic outgassing.
7. The salt added by rivers is continuously removed at the same rate it is added. Precipitation as sediment (biogenous and hydrogenous) accomplishes the removal.
8. Residence time is the average time a substance stays in a reservoir before leaving it.
9. 2800kg / 700 kg / year = 4 years
10. Less reactive ions have longer residence times.
11. If an ion has a residence time far greater than the ocean’s mixing time (1000 years), it will obey the principle of constant proportions.
12. Conservative constituents would obey the principle of constant proportions because they do not change in concentration except through surface processes that add or remove water. If only water is added or removed from a salt solution, then the concentration of salt will change, but not the relative proportions of the ions that make up that salt to one another.
13. Since salinity’s components obey the principle of constant proportions, if you know the concentration of one ion (i.e. Chloride) and it’s proportion relative to all the other ions (a constant), you can just multiply it’s concentration by a known constant to determine the total concentration of dissolved ions (=salinity).
14. Plot the typical graphs of dissolved oxygen and carbon dioxide vs. depth; and indicate the reasons for the high and low spots in these curves (see class notes and figure 6.8).
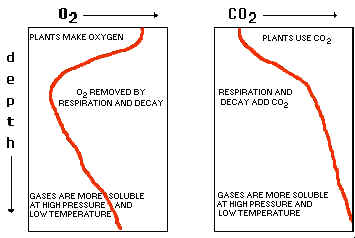
15. Gases are more soluble when temperatures are low but pressures are high.
16.
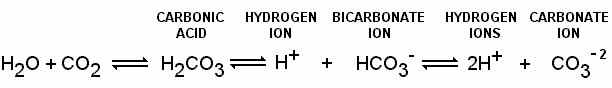
8800 Grossmont College Drive
El Cajon, California 92020
619-644-7000
Accessibility
Social Media Accounts