FORMAL CHARGE AND RESONANCE
Formal charge is an accounting procedure. It allows chemists to determine the location of charge in a molecule as well as compare how good a Lewis structure might be. The formula for calculating formal charge is shown below:
![]()
You should be able to determine which atoms have formal charge based on
comparing the structure with common, known neutral structures. To do this you
need to recognize the common neutral structures: C 4 bonds, N 3 bonds, 1 lone
pair, O 2 bonds, 2 lone pairs, F 1 bond, 3 lone pairs.
Consider the molecule H2CO2. There are two possible Lewis structures for this molecule. Each has the same number of bonds. We can determine which is better by determining which has the least formal charge. It takes energy to get a separation of charge in the molecule (as indicated by the formal charge) so the structure with the least formal charge should be lower in energy and thereby be the better Lewis structure.
|
|
|
|
|
|
The two possible Lewis structures are shown below. They are connected by a double headed arrow and placed in brackets. The non-zero formal charge on any atoms in the molecule have been written near the atom.
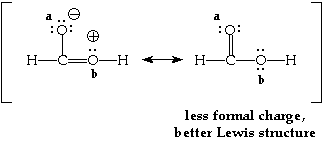
The Lewis structure for certain molecules or ions can be drawn in more than one way.
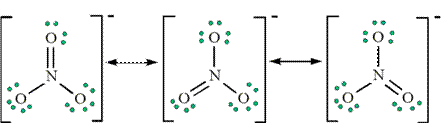
e.g. NO2 - number of e- = 5+ 2 ( 6 ) +1 = 18 e- (or 9 pairs).
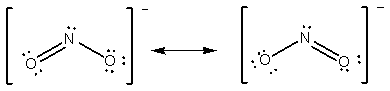
You would expect that the doubly bonded oxygen-nitrogen distance to be slightly less than the singly bonded distance. Actually, both N-O distances are equivalent. The true structure of the molecule is some combination of the two. Anytime you have more than one valid structure for a molecule or ion, you have what are known as resonance structures. If you have many possible resonance forms, you choose the most likely resonance form or forms by calculating the formal charge on each atom in each resonance form.
By convention, resonance forms are shown connected by "double-headed arrows" to stress the fact that they are not separate valence isomers in some sort of rapid equilibrium.
Rules for Resonance
Although we cannot use drawings on paper to adequately represent molecules with resonance, drawing resonance forms allows to make predictions and helps us understand the reactivity of organic chemical compounds
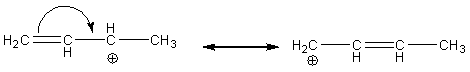
We Can Never Change the Connectivity of the Atoms of the Compound
When Moving Electrons:
· The electron(s) moved must remain in association with at least one of the atoms with which it (they) was associated with before the move
· For 2-electron moves, you essentially make a double bond from an unshared pair, an unshared pair from a double bond, or move the position of a double bond
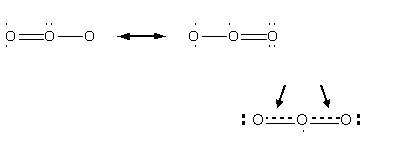
Resonance Structures for Ozone
Are these resonance structures equivalent? YES
Note: We would expect that the bond lengths in the NO3- ion to be somewhat shorter than a single bond
More Important Resonance Structures:
· Have formal charges on all atoms are at or near 0
· Structures which involve the generation and separation of charge contribute less than neutral structures, or structures with the same charge,
· Structures with negative charges on electronegative atoms contribute more than structures with negative charges on more electropositive atoms.

