Author:Welker
Monosaccharides cannot be hydrolyzed into simpler molecules. (Disaccharides can be hydrolyzed to two monosaccharides, etc.) Monosaccharides contain one carbon in the carbonyl oxidation state as either an aldehyde (aldose) or ketone (ketose). All other carbons contain alcohol functional groups. If the carbohydrate contains a total of five carbons, it is called a pentose. A carbohydrate that contains six total carbons is a hexose.
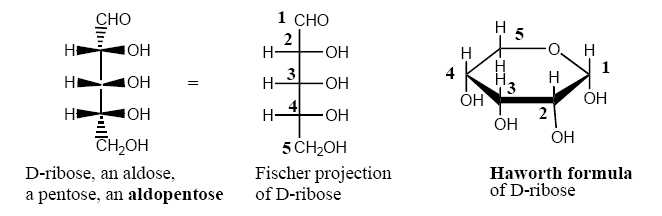
Carbohydrates exist in aqueous solution largely as five or six member ring hemiacetals. Five member ring sugars are called furanoses (related to furan) and six member ring sugars are called pyranoses (related to pyran.)
Example of D-ribose cyclization in solution to pyanose and furanose forms:
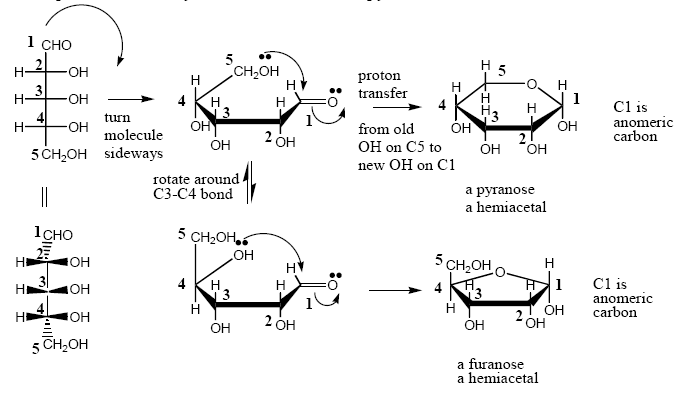
D (R) or L (S) refers to the stereochemistry of the chiral center furthest from the carbonyl group. The carbohydrate (sugar) in the previous example is a D-carbohydrate because the stereochemistry at C4 is R.
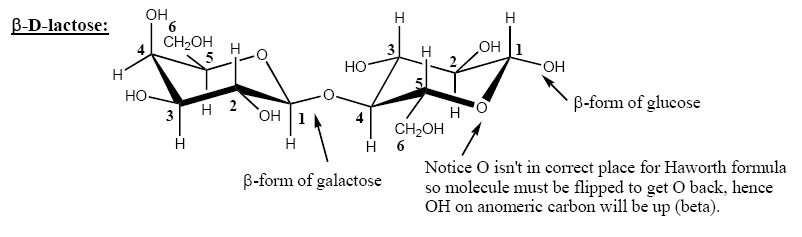
In the furanose and pyranose forms, the only carbon with two C-O bonds is called the anomeric carbon (C1 in aldoses.) The OH on the anomeric carbon can either be up or down depending on the geometry of the molecule as it cyclizes. By convention, sugar chemists always draw the Haworth projection of a sugar with the O of the ring in the same position, as shown in this document and in your texts. In the conventional Haworth projection, if the OH on the anomeric carbon is down, the carbohydrate is the alpha (α) anomer. If the OH on the anomeric carbon is up, the carbohydrate is the beta (β) anomer.1
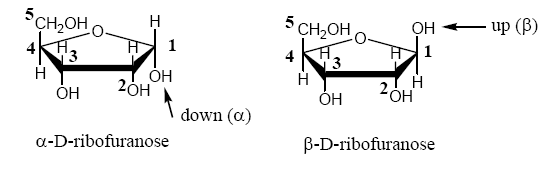
Aldoses are always numbered so that the anomeric carbon of each saccharide is C1. Number remaining carbons around each ring.
Furanose and pyranose forms of carbohydrates are hemiacetals. If the anomeric OH is converted to an OR, the carbohydrate is no longer a hemiacetal but an acetal. The acetal of a carbohydrate is called a glycoside.
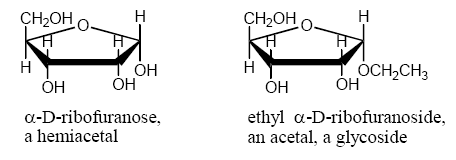
1 Determination of α versus β anomers: for the cyclic form of a D-sugar, drawn with the O of the ring back and the anomeric C on the right side of the molecule, if the anomeric OH is up, it’s the beta form. If the anomeric OH is down, it’s the alpha form.
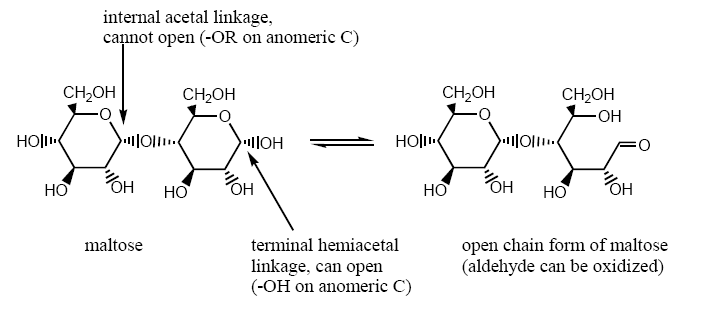
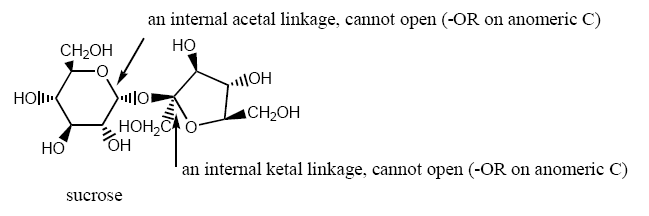
Hemiacetals are in equilibrium with the open chain form of the carbohydrate in solution. Aldehydes can be oxidized to carboxylic acids in the open chain form. Since the sugar can be oxidized, it is called a reducing sugar. (The sugar reduces something else as it is oxidized.) Glycosides are not in equilibrium with the open chain form. Since glycosides cannot open, they cannot have a carbonyl group, and they cannot be oxidized. Glycosides are nonreducing sugars. In summary, carbohydrates with an OH on the anomeric carbon are reducing sugars. Carbohydrates with OR on the anomeric carbon are nonreducing sugars.
Maltose: a reducing sugar (and a disaccharide)
Sucrose: a nonreducing sugar (and a disaccharide)
8800 Grossmont College Drive
El Cajon, California 92020
619-644-7000
Accessibility
Social Media Accounts